Deficits in mitochondrial TCA cycle and OXPHOS precede rod photoreceptor degeneration during chronic HIF activation
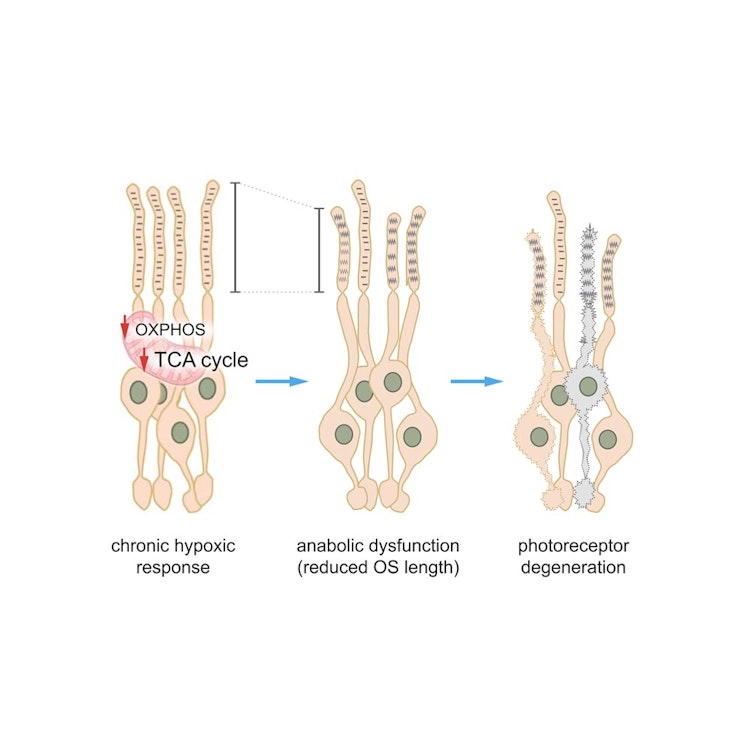
Background Major retinal degenerative diseases, including age-related macular degeneration, diabetic retinopathy and retinal detachment, are associated with a local decrease in oxygen availability causing the formation of hypoxic areas affecting the photoreceptor (PR) cells. Here, we addressed the underlying pathological mechanisms of PR degeneration by focusing on energy metabolism during chronic activation of hypoxia-inducible factors (HIFs) in rod PR. Methods We used two-photon laser scanning microscopy (TPLSM) of genetically encoded biosensors delivered by adeno-associated viruses (AAV) to determine lactate and glucose dynamics in PR and inner retinal cells. Retinal layer-specific proteomics, in situ enzymatic assays and immunofluorescence studies were used to analyse mitochondrial metabolism in rod PRs during chronic HIF activation. Results PRs exhibited remarkably higher glycolytic flux through the hexokinases than neurons of the inner retina. Chronic HIF activation in rods did not cause overt change in glucose dynamics but an increase in lactate production nonetheless. Furthermore, dysregulation of the oxidative phosphorylation pathway (OXPHOS) and tricarboxylic acid (TCA) cycle in rods with an activated hypoxic response decelerated cellular anabolism causing shortening of rod photoreceptor outer segments (OS) before onset of cell degeneration. Interestingly, rods with deficient OXPHOS but an intact TCA cycle did not exhibit these early signs of anabolic dysregulation and showed a slower course of degeneration. Conclusion Together, these data indicate an exceeding high glycolytic flux in rods and highlight the importance of mitochondrial metabolism and especially of the TCA cycle for PR survival in conditions of increased HIF activity.
Download
todorova_2023.pdfResearchers





